The system Al2O3-P2O5-H2O at temperatures below 200 °C: Experimental data on the stability of variscite and metavariscite AlPO4·2H2O | Semantic Scholar

PDF) Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature | Federico Nores Pondal - Academia.edu

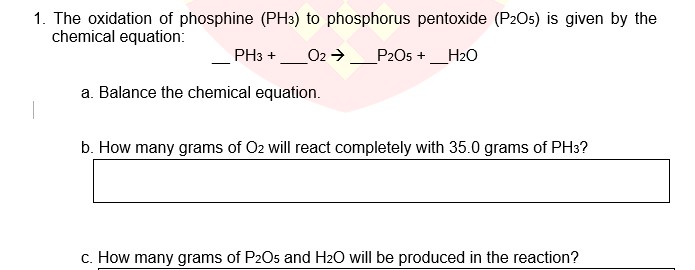
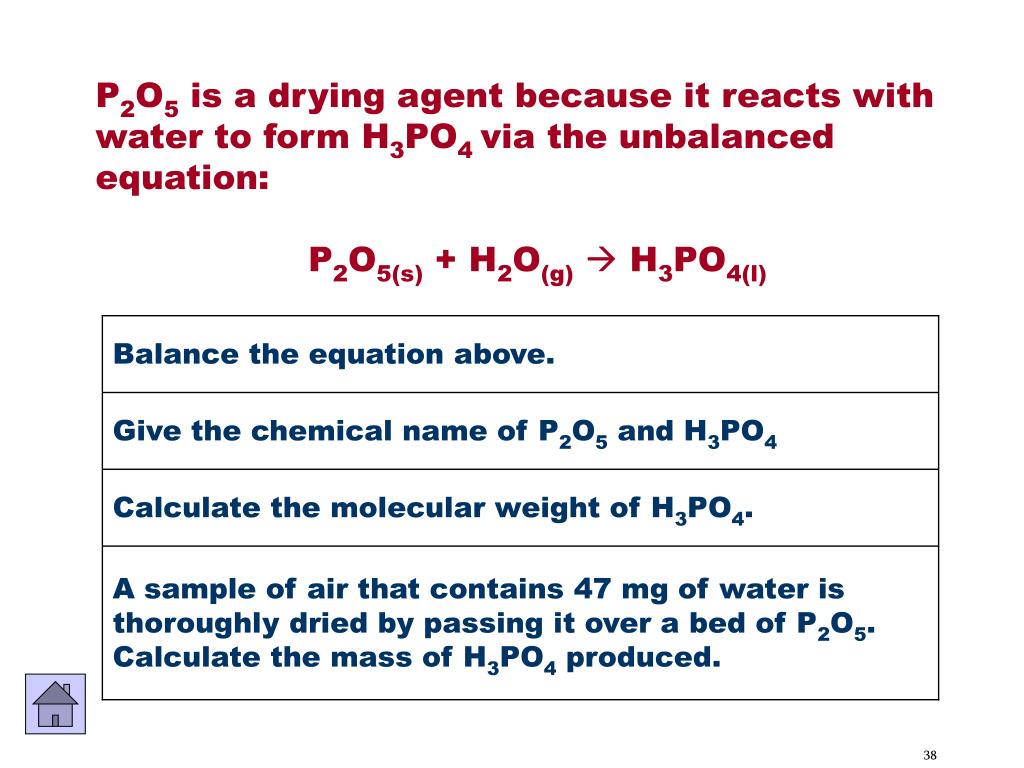
SOLVED: The oxidation of phosphine (PH3) to phosphorus pentoxide (P2O5) is given by the chemical reaction.PH3 +O2 —> P2O5 + H2Ob. How many grams of O2 will react completely with 35.0 grams
![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/4-Figure2-1.png)
PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar

Total Ni, exchangeable ions, available P2O5, and pH (H2O) in the soils... | Download Scientific Diagram

Solubility study at high phosphorus pentoxide concentration in ternary system CaCO3+P2O5+H2O at 25, 35 and 70 °C - ScienceDirect
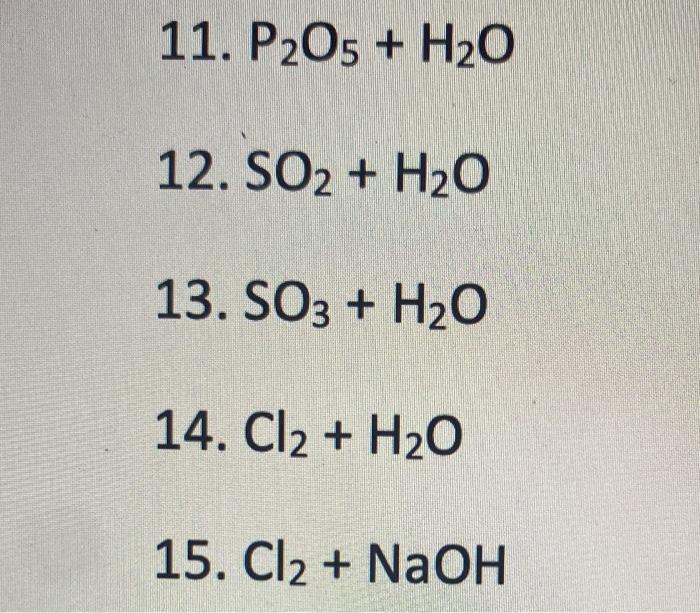
ntP2O5 on treatment with exces s of H2O followed by excess of NH4OH forms (NH4)2HPO4. If hundred gram of (NH4)2HPO4 is formed then find out the mass of p 2 o5 initial