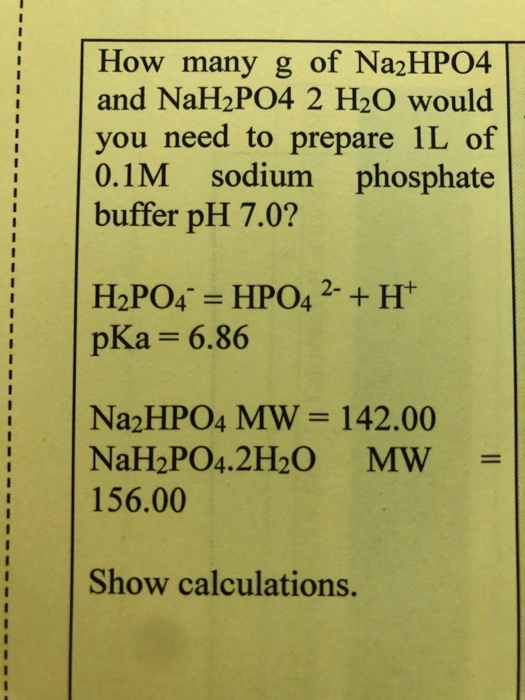
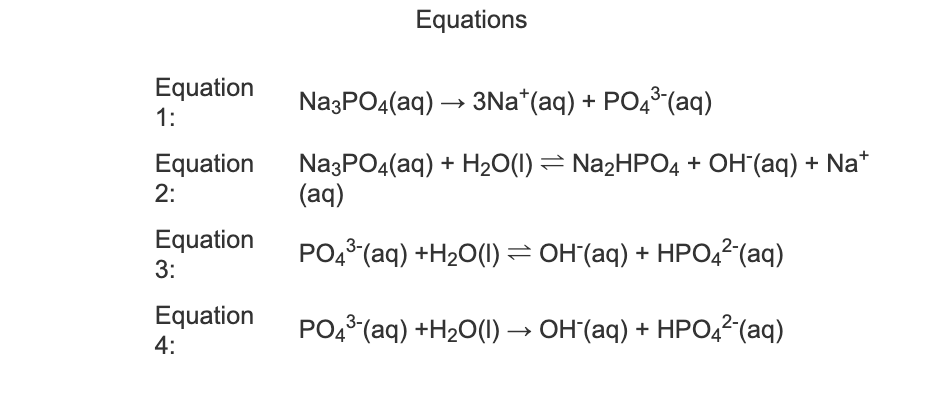
SOLVED: What is the balanced equation and the net ionic equation. NaH2PO4 + Na2HPO4 + H2O + MgCl2 ⇒ NaH2PO4 + Na2HPO4 + H2O + NaCl ⇒ ( we did an experiment

1 Bán Hóa chất di-Sodium hydrogen phosphate dihydrate, - Na2HPO4.2H2O - SO0339 - Scharlau giá rẻ ở hcm

Measurement and Modeling of the Solubility of NH4VO3 in the Na2HPO4–H2O and (NH4)2HPO4–H2O Systems | Journal of Chemical & Engineering Data

For the equilibrium SrCl2· 6H2O(s) SrCl2· 2H2O(s) + 4H2O(g) the equilibrium constant Kp = 16 × 10^-12 atm^4 at 1^0 C. If one litre of air saturated with water vapour at 1^0
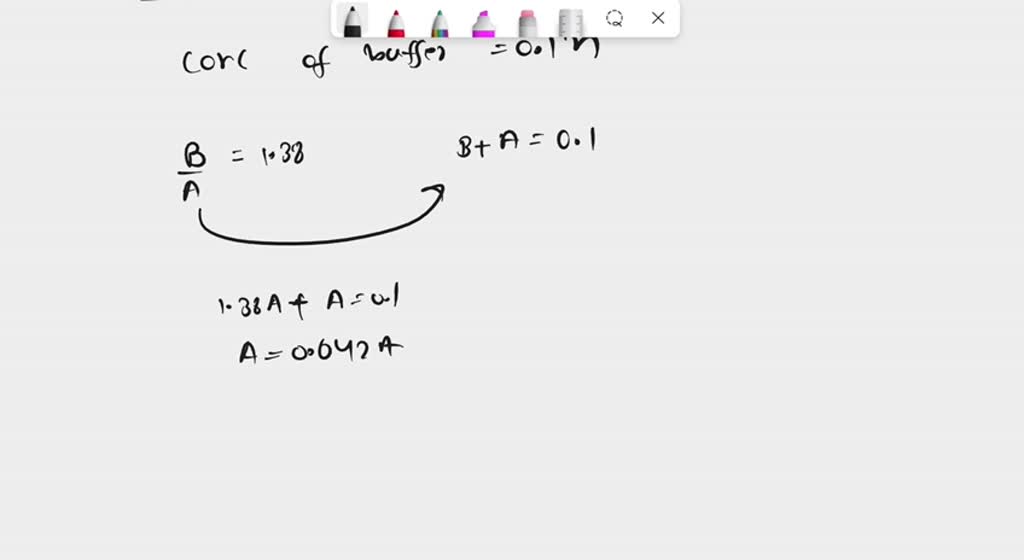


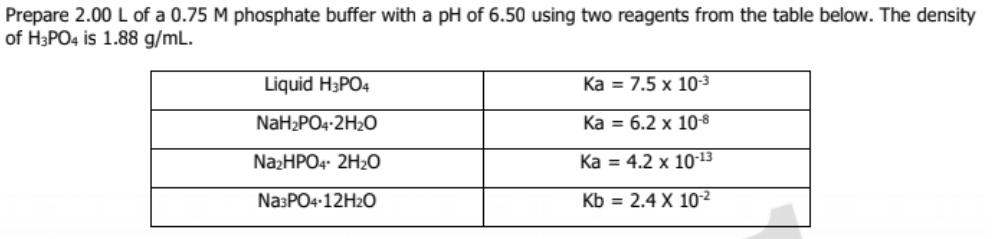



![Sodium Phosphate Dibasic Dihydrate [Na2HPO4.2H2O] Molecular Weight Calculation - Laboratory Notes Sodium Phosphate Dibasic Dihydrate [Na2HPO4.2H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/01/sodium-phosphate-dibasic-dihydrate-molecular-weight-calculation-300x186.jpg)