Lithium Hexamethyldisilazide-Mediated Enolization of Acylated Oxazolidinones: Solvent, Cosolvent, and Isotope Effects on Competi

Cyclic Poly(α-peptoid)s by Lithium bis(trimethylsilyl)amide (LiHMDS)-Mediated Ring-Expansion Polymerization: Simple Access to Bioactive Backbones | Journal of the American Chemical Society

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect

Regiospecificity in Ligand-Free Pd-Catalyzed C–H Arylation of Indoles: LiHMDS as Base and Transient Directing Group | ACS Catalysis

Reaction of Ketones with Lithium Hexamethyldisilazide: Competitive Enolizations and 1,2-Additions | Journal of the American Chemical Society

Scheme 55. Reagents: i, LiHMDS, THF, –78ºC; ii, oiodobenzaldehyde ;... | Download Scientific Diagram

Figure 2 from Structure of Lithium Hexamethyldisilazide (LiHMDS) in the Presence of Hexamethylphosphoramide (HMPA). Spectroscopic and Computational Studies of Monomers, Dimers, and Triple Ions. | Semantic Scholar

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect

Scheme 15. Synthesis of AM281 (74). Reagents and conditions: (a) (i)... | Download Scientific Diagram
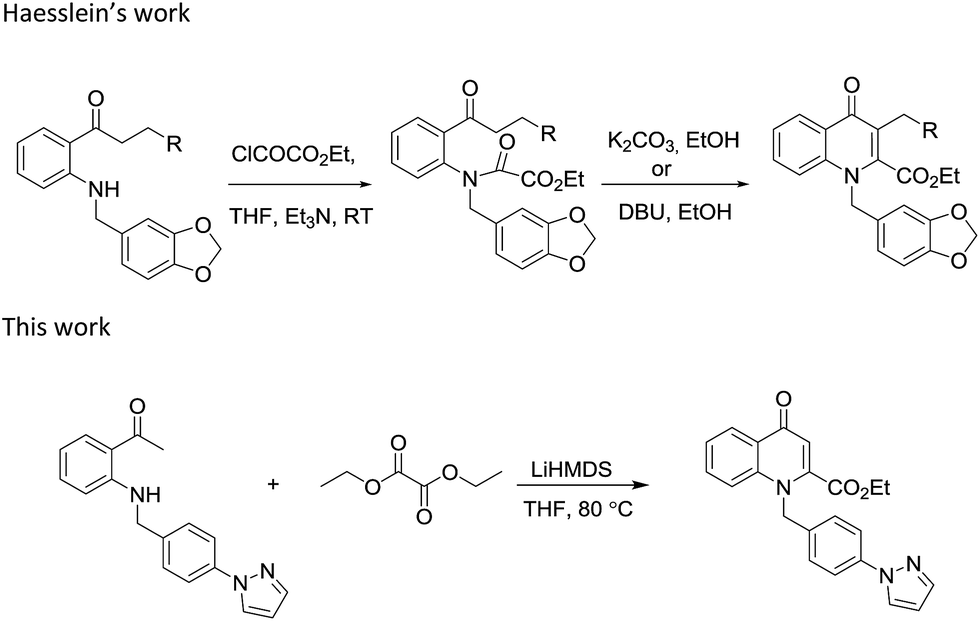
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C

Scheme 34 Reagents and condition—a (i) LiHMDS, THF, −78 °C; (ii) R 2 CH... | Download Scientific Diagram
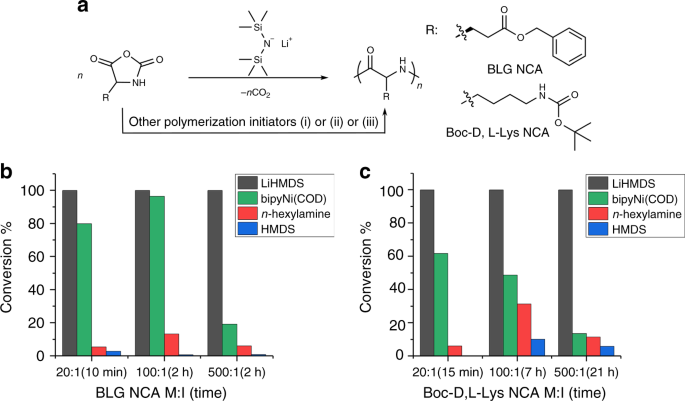
Lithium hexamethyldisilazide initiated superfast ring opening polymerization of alpha-amino acid N-carboxyanhydrides | Nature Communications





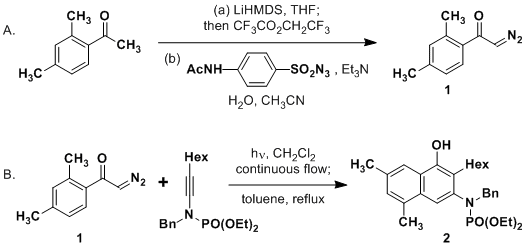
amide/LiHMDS_Ex_01.png)

