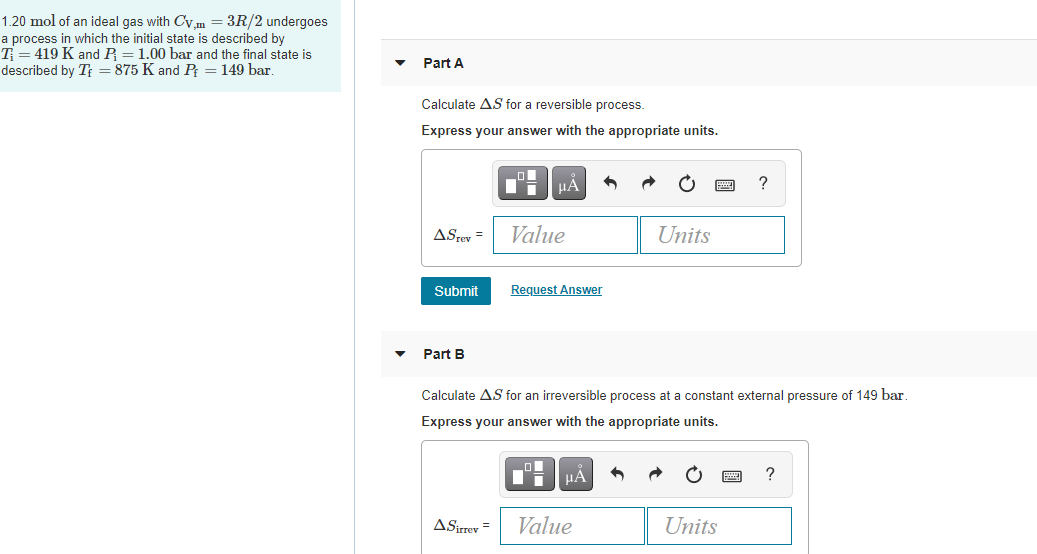
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download

CV curves in 1.0 mol L −1 ethanol and 1.0 mol L −1 KOH with a sweep... | Download Scientific Diagram

CV curves of PPGN-n in 0.1 mol L À1 KOH solution (a) and in 1 mol L À1... | Download Scientific Diagram

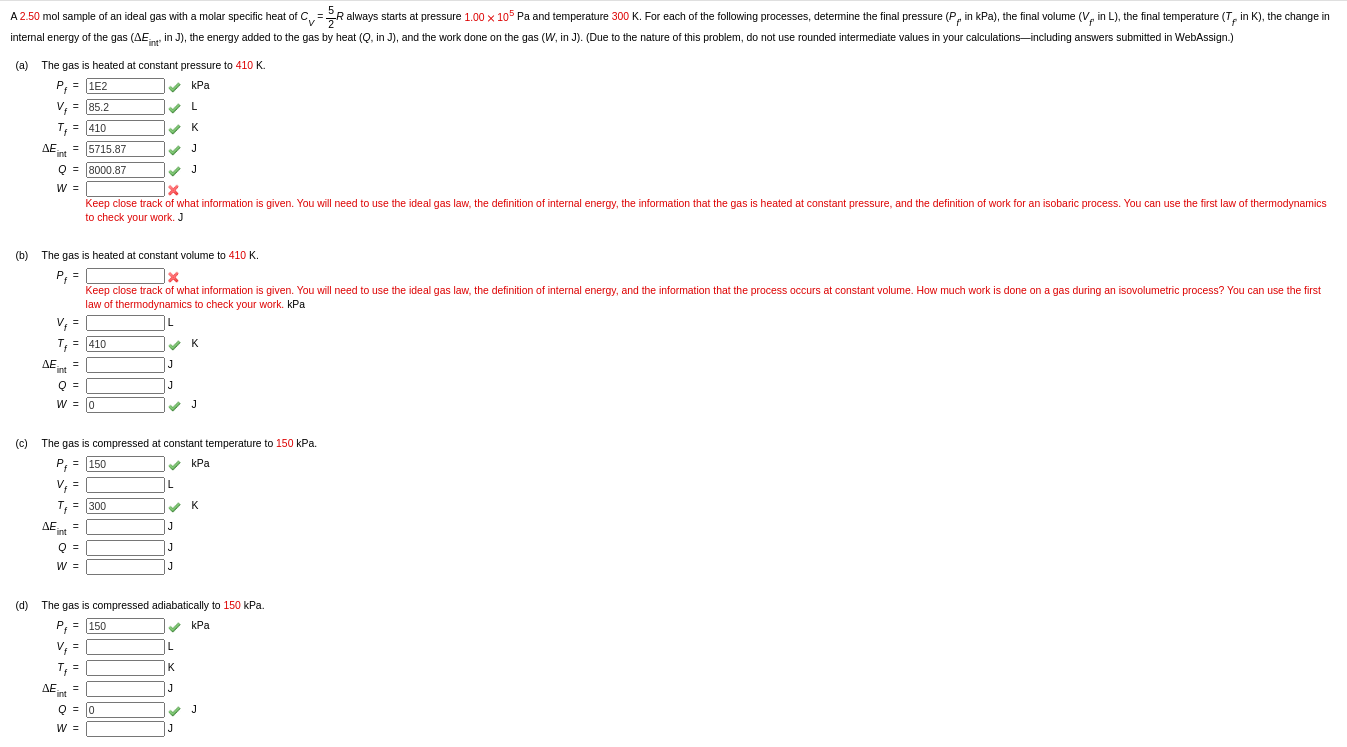
SOLVED: For an ideal gas CV and Cp are different because of the workW associated with the volume change for a constant-pressure process.To explore the difference between CV and Cp for a

For 1 mol of a triatomic ideal gas C(v) = 3R (R is universal gas constant). Fid delta (=C(p)//C(v)) for that gas.

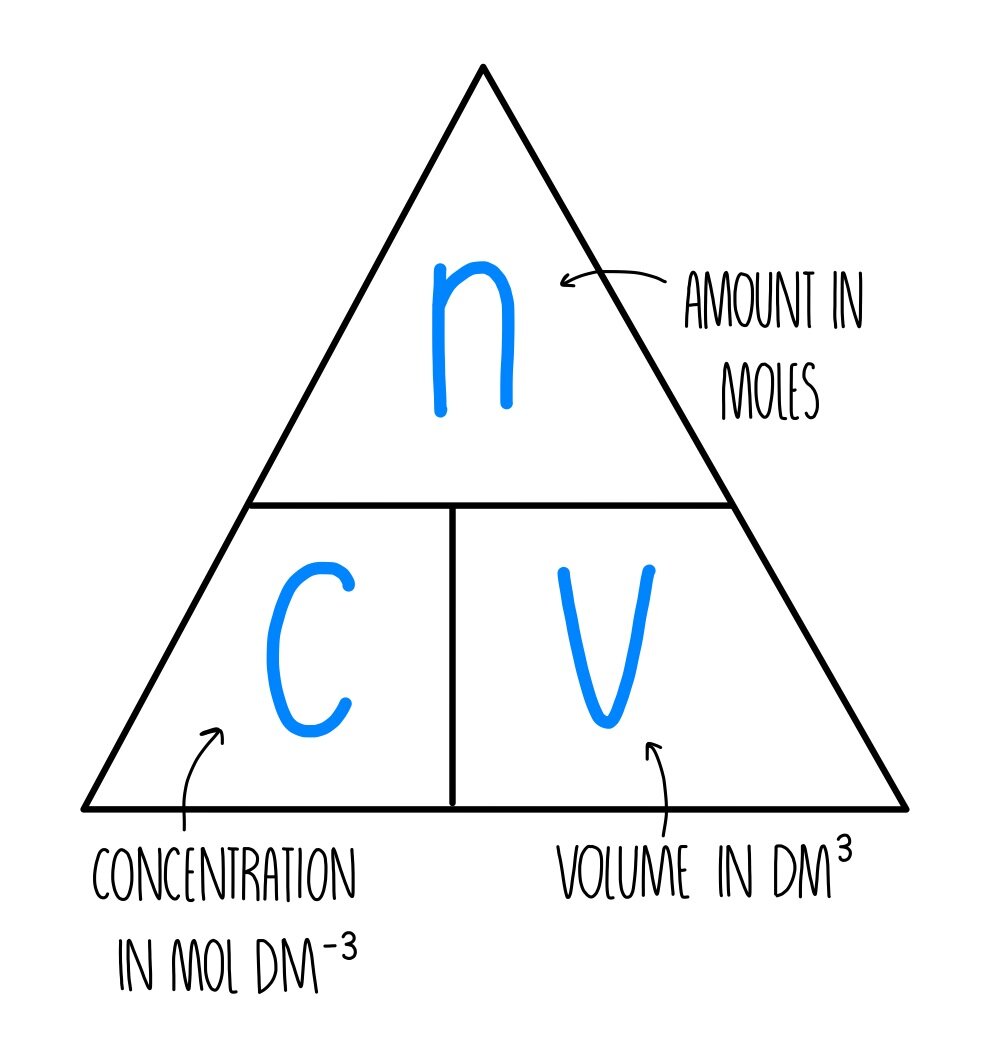
The Mole And Concentration Formula Triangle Isolated On White Relationship Between Concentration Moles And Volume Cnv Stock Illustration - Download Image Now - iStock
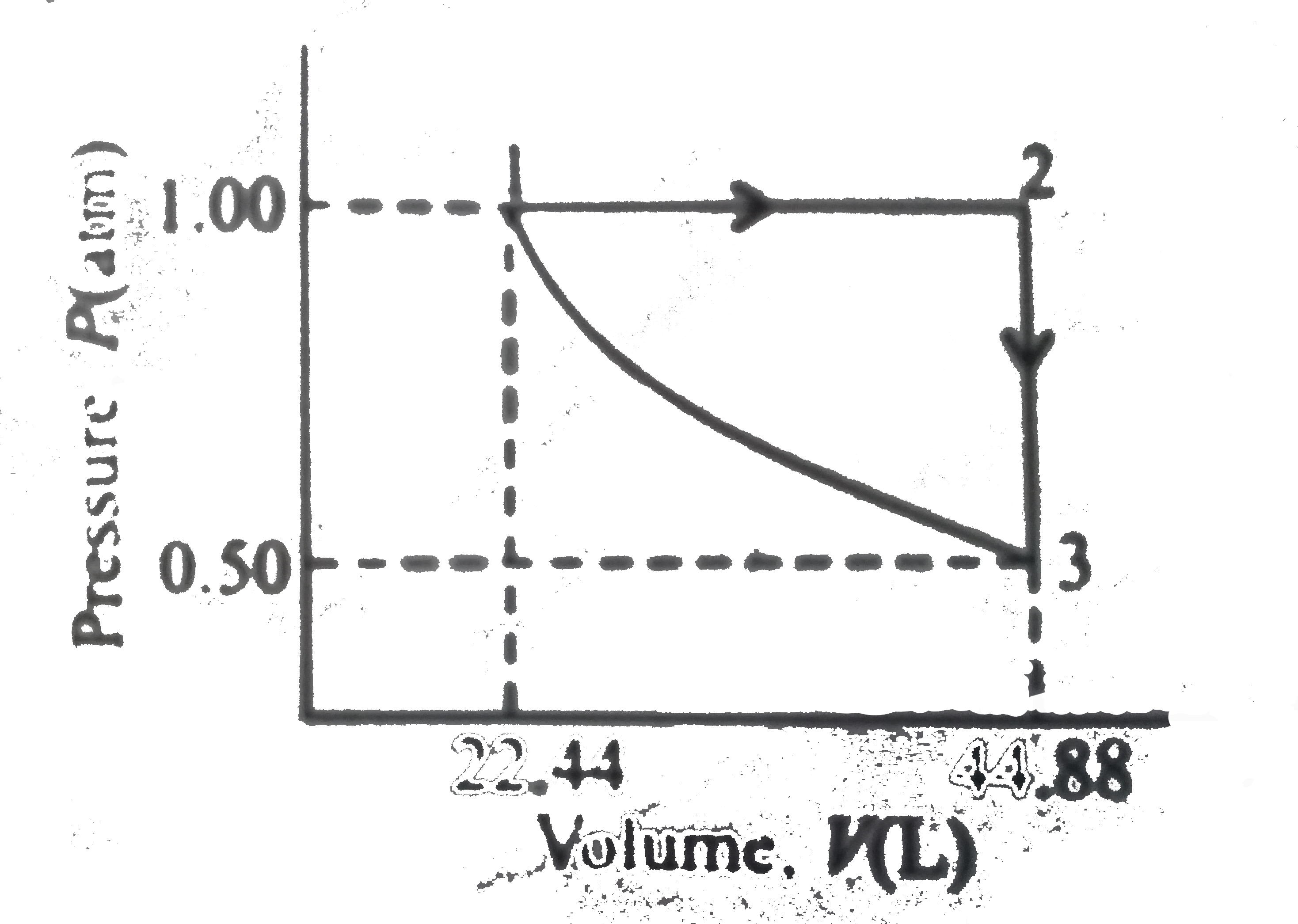
A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(2)R) is taken through the cycle as shown. Temperature at points (1),(2) and (3) respectively is

A) CV curves of 0.04 mol L À 1 BR buffer (pH 4.0) with 5 % of DMSO (À... | Download Scientific Diagram

a Comparison of CV measurements recorded in (LiCl–KCl)(eut.)–0.09 mol%... | Download Scientific Diagram



![N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube](https://i.ytimg.com/vi/rh4IuW1fP6g/hqdefault.jpg)