Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine | Nature Communications

XRD pattern of (a) pure PVP and PVP doped with (b) 10 mol% (c) 15 mol%... | Download Scientific Diagram

Bonide MOLEMAX Mole & Vole Repellent Granules, 10 lbs. Ready-to-Use, Outdoor Lawn & Garden Mole Control, People & Pet Safe

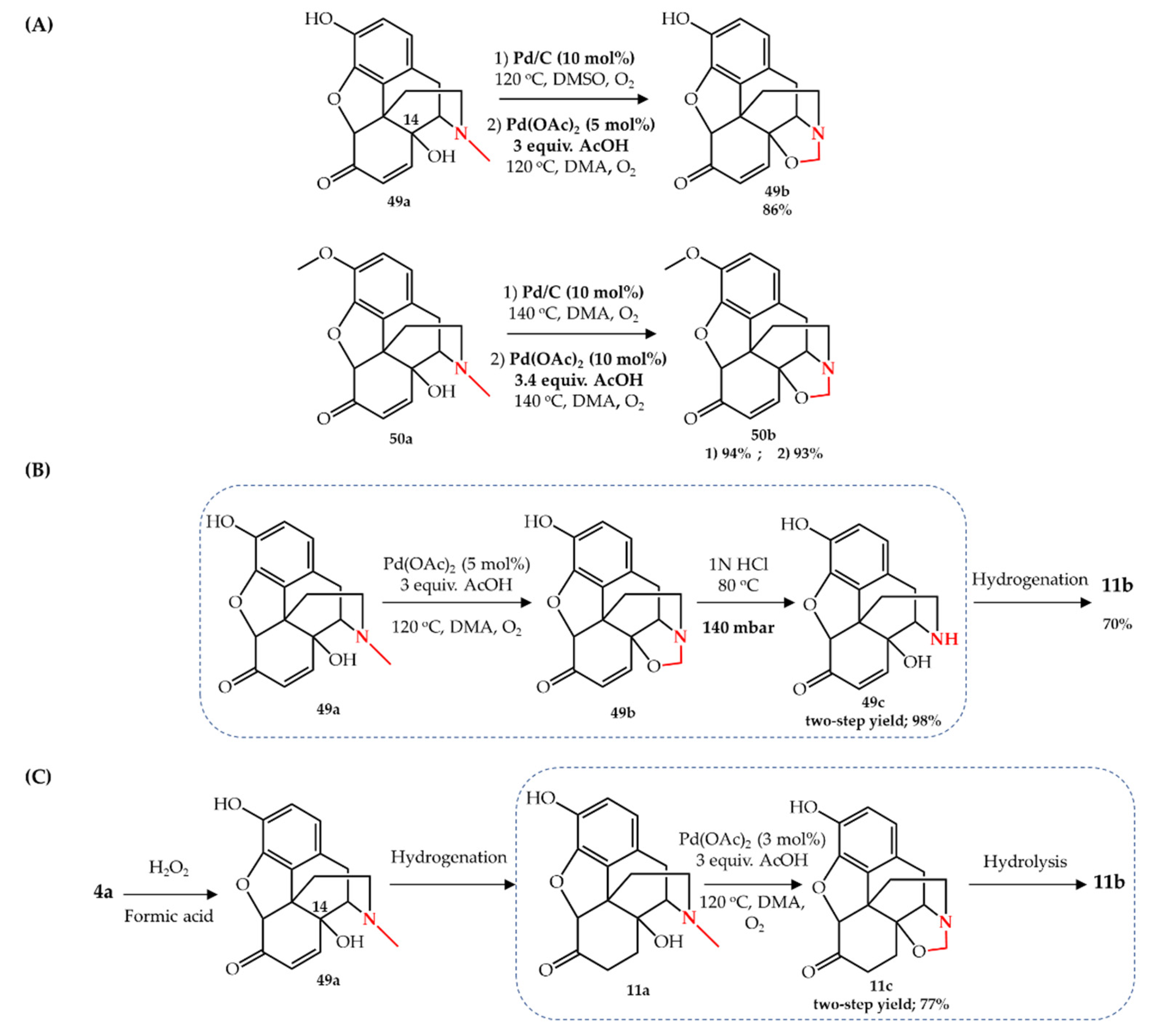
A Change from Kinetic to Thermodynamic Control Enables trans-Selective Stereochemical Editing of Vicinal Diols | Journal of the American Chemical Society
![For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] = For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =](https://d1hhj0t1vdqi7c.cloudfront.net/v1/TVhJa2FqVWNSMFk=/sd/)
For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =
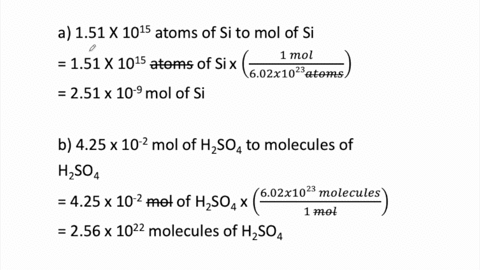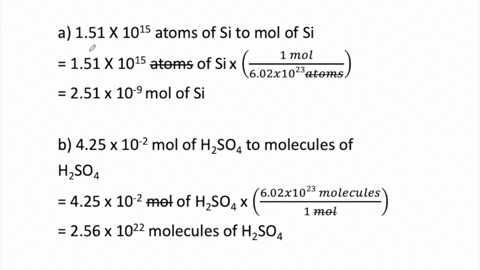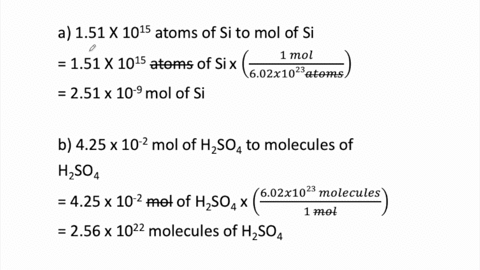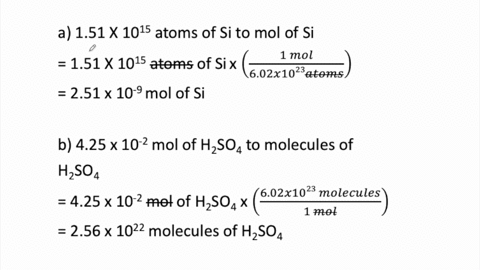
SOLVED:Determine the number of moles in each substance. a. 3.25 ×10^20 atoms of lead b. 4.96 ×10^24 molecules of glucose c. 1.56 ×10^23 formula units of sodium hydroxide d. 1.25 ×10^25 copper (II) ions

SOLVED: a mole of sodium atoms contains 6.02 x 10^23 atoms. How many moles would be needed in order to have 2.50 x 10^24 atoms?
123 The molar solubility of CaF2 (Ks5.3 x10 11) in 0.1 M solution of NaF will be Co5.3 x 10 11 mol L 1(2) 5.3 × 10 8 mol L 1A) E) S.3 × 10 9 mol L 1くが(141) 5.3 × 10 10 mol L 1"卡

Each mole of substance A (Molar mass =720 ) required 10 moles of water for complete hydrolysis and - YouTube
What are some important formulas for mole concepts, like how to find the number of atoms, etc.? - Quora
![Solubility product of AgCl is 1.8 × 10^-10 . Calculate its molar solubility and solubility in g/L. [Molar mass of AgCl is 143.5 g mol^-1 ] Solubility product of AgCl is 1.8 × 10^-10 . Calculate its molar solubility and solubility in g/L. [Molar mass of AgCl is 143.5 g mol^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/5064616/add4819a-6281-4659-ab5c-8471f2633afa.jpg)